client
Lohmann & Rauscher GmbH & Co. KG
location
Neuwied
services
Master planning of a biotechnological production and Integrated Project Delivery as general contractor for building, technical building equipment and production facilities.
area
approx. 1.700 m² GFA | 19.000 m³ GV
Project Term
2005 - 2008
THE CLIENT
Lohmann & Rauscher GmbH & Co. KG is a leading international supplier of future-oriented medical and hygiene products, with headquarters in Rengsdorf (D) and Vienna (AT). Lohmann & Rauscher manufacture, among others, collagen wound dressings for hard-to-heal wounds from natural raw materials.
THE TASK
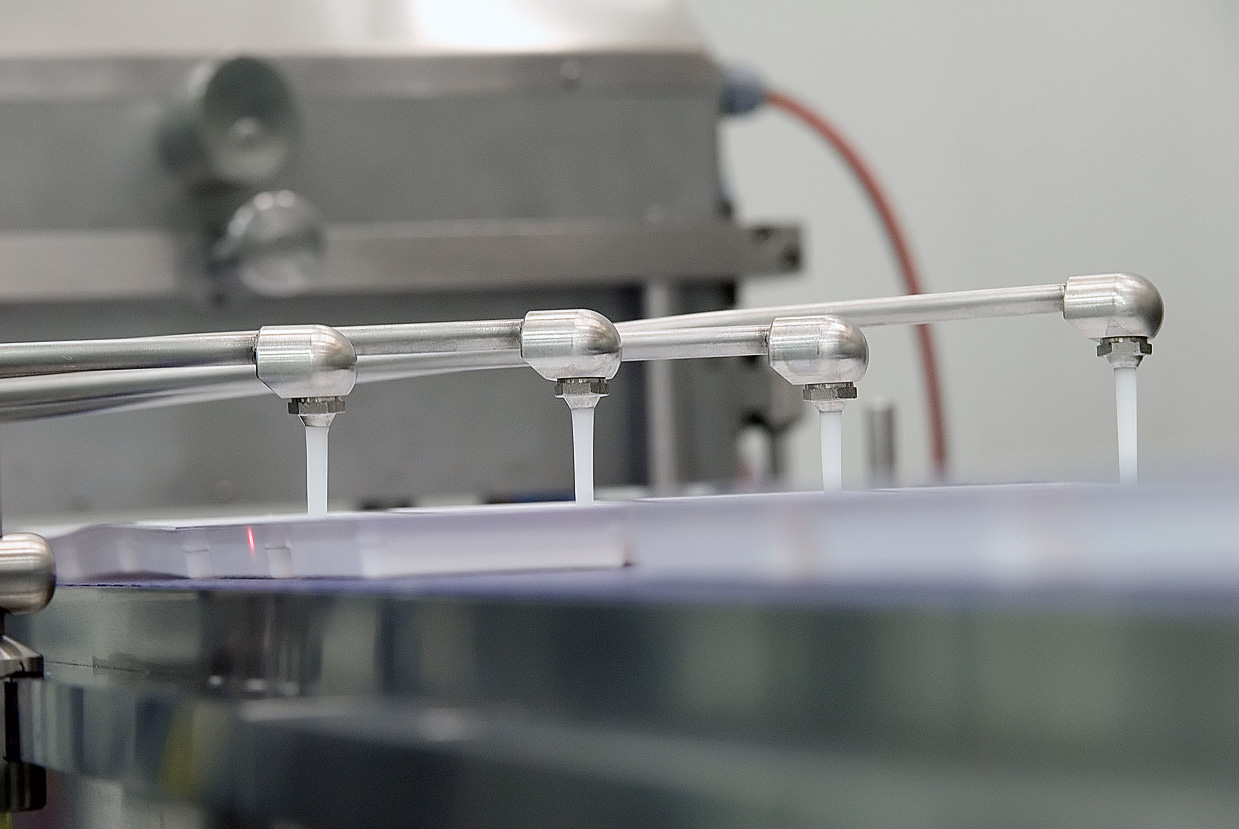
Due to the great success of this innovative product, considerable capacity expansions were necessary and the production process used until then required automation and scale-up. Afte the evaluation of various alternatives, the client decided to build a new aseptic collagen production and packaging facility. The solution was integrated into an existing warehouse as a “house-in-house” concept.
THE PLANNING PROCESS
The profitability analyses of the production expansion brought the project under high cost pressure. In addition, conditions prevailing at the location were cramped. Therefore, a concept had to be developed that not only met the tight budget but also fulfilled the requirements for aseptic production. The solution was worked out in workshops in close cooperation between the client’s experienced staff and the contractor’s experts. In order to obtain cost security, the client decided to award the planning and implementation to the contractor.
THE REALISATION PROCESS
Within the international marketing of the product and a maximum flexibility in the production of other medical devices and pharmaceuticals, a new production facility was built, taking into account all legal requirements for aseptic production (GMP & FDA compliance). They were integrated into an existing production hall at the Neuwied site.
THE CHALLENGE
The main challenge was to integrate the semi-automated production steps with high-capacity equipment into an existing warehouse while meeting the requirements of aseptic production. All this required a parallel organisational development on the part of the client.